Lanoxin 125 Mcg Tablet
Digoxin oral tablet is used to treat atrial fibrillation mild to moderate heart failure in adults and heart failure in children.

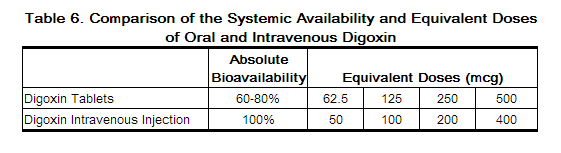
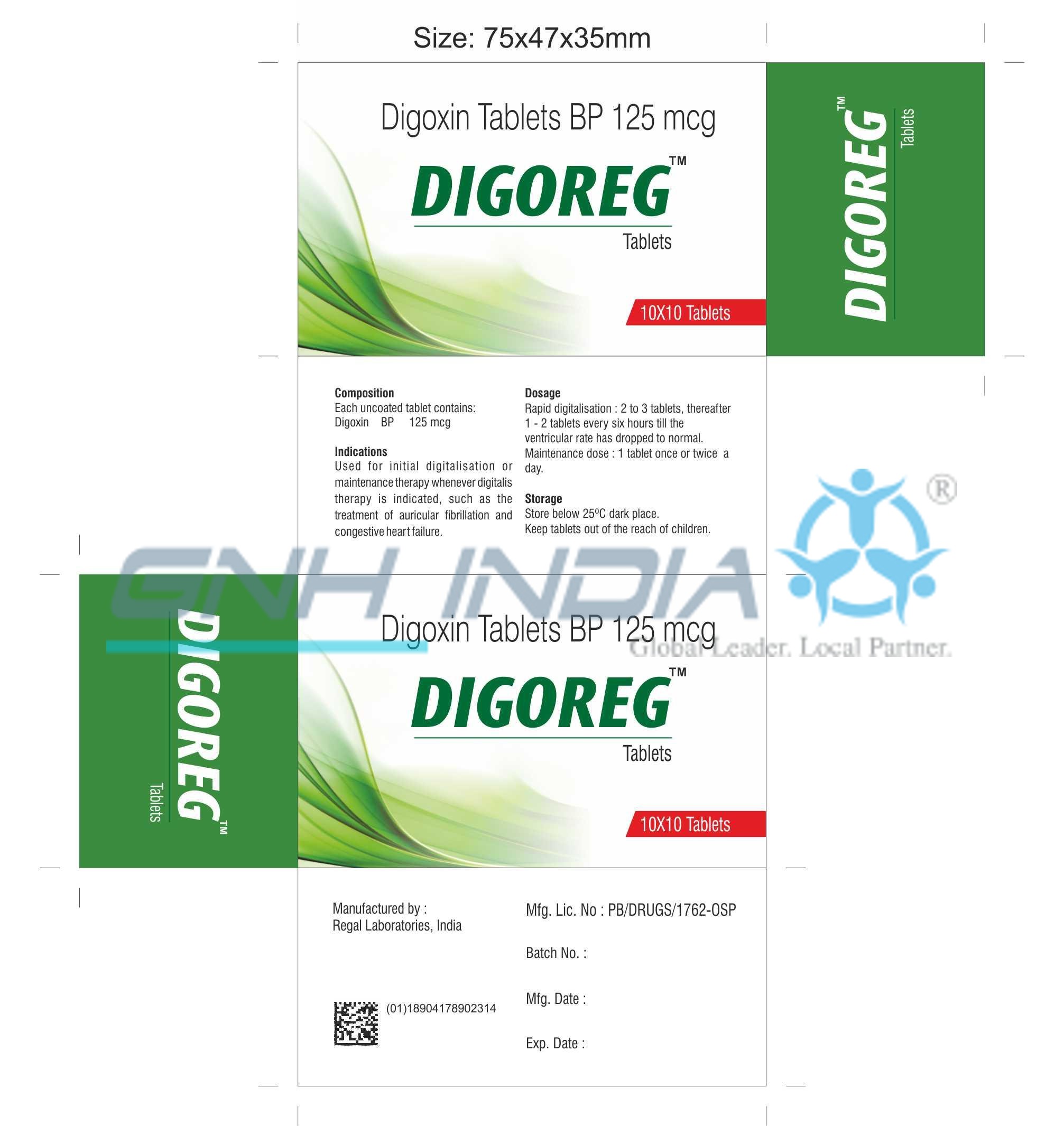
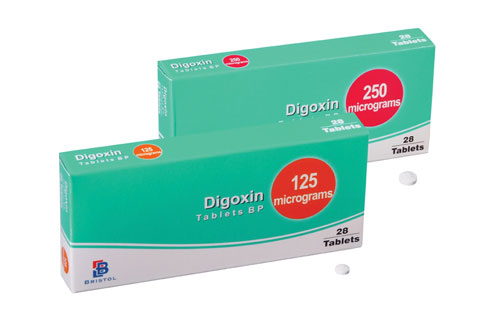
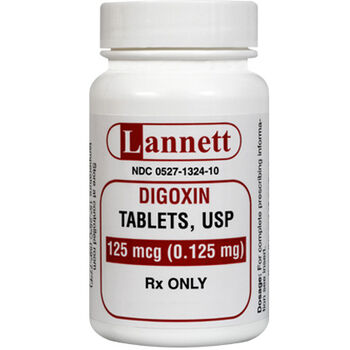
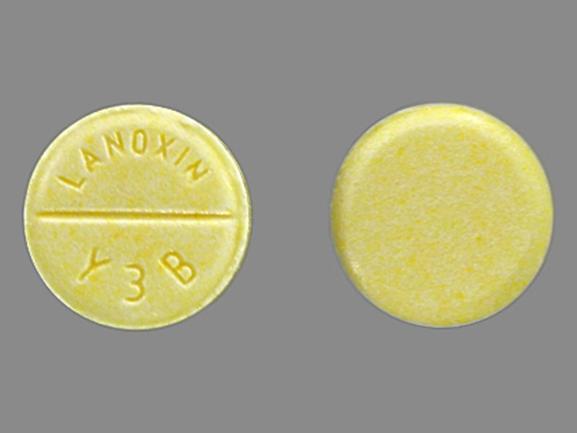
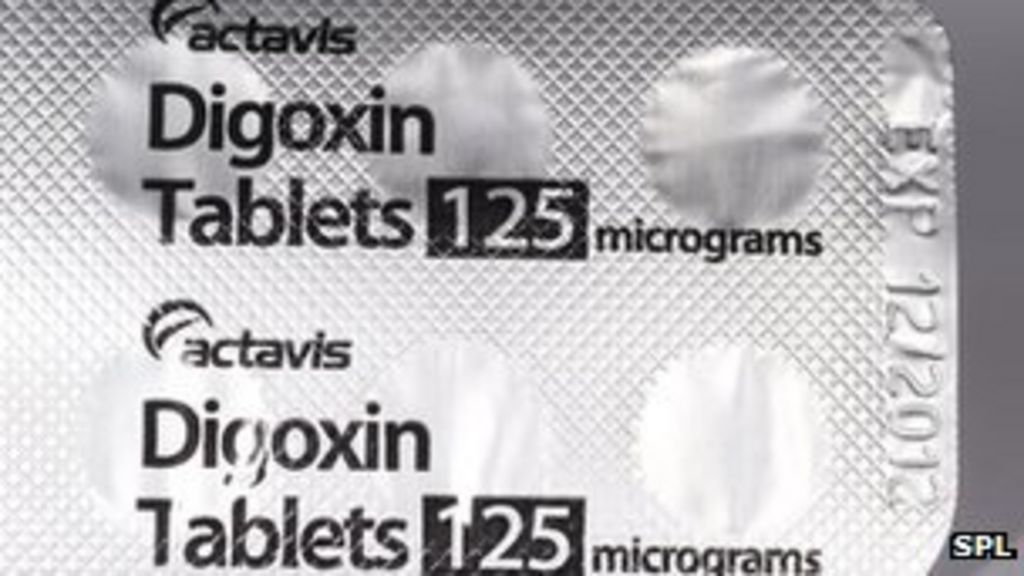
Lanoxin 125 mcg tablet. 125 mcg 0125 mg each scored tablet contains 125 mcg 0125 mg digoxin. Lanoxin tablets are supplied as 125 or 250 mcg microgram once a day. The other ingredients are lactose maize starch rice starch maize starch hydrolysed and magnesium stearate.
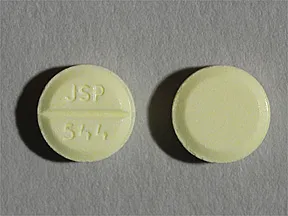
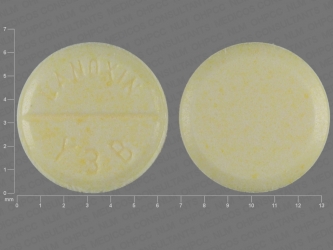
Sometimes the doses may be written in micrograms mcg. 30 to 45 mcgkg once a day comments im administration is not recommended due to associated pain and muscle necrosis. Pill with imprint lanoxin y3b is yellow round and has been identified as lanoxin 125 mcg 0125 mg.
The usual amount of tablets that a 70 kg patient requires to achieve 8 to 12 mcgkg peak body stores is 750 to 1250 mcg. 120 mcg and 250 mcg. Adequate dosing without toxicity is determined usually by a blood test that determines the drugs level in the blood.
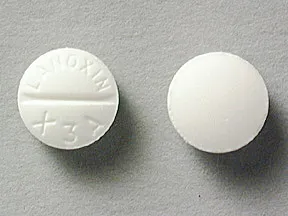
What lanoxin looks like and contents of the pack lanoxin is a white round flat tablet debossed d012 on the one side and plain on the other side. It is supplied by glaxosmithkline. Your doctor will draw blood to determine how much.
Tablets are available in doses of 0125 milligram mg and 0250 mg. Lanoxin digoxin tablets usp. 10 to 15 mcgkg maintenance dose iv.
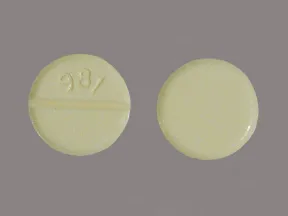
Color yellow shape round imprint 437 this medicine is a yellow round scored tablet imprinted with 437. Back to gallery. Heart failure and belongs to the drug classes group v antiarrhythmics inotropic agents.
Risk cannot be ruled out during pregnancy. 24 to 36 mcgkg once a day tablets. What lanoxin contains each tablet contains 0125 mg 125 micrograms of digoxin.
A very serious allergic reaction to this drug is rare. Enlargedtender breasts in men. This unit dose packaging is intended for institutional inpatient use.
Tell your doctor right away if you notice any unusually fastslowirregular heartbeat. Lanoxin is used in the treatment of atrial fibrillation. Digoxin 125 mcg 0125 mg tablet.
Digoxin 250 mcg 025 mg tablet. 34 to 51 mcgkg once a day oral solution. 8 to 12 mcgkg tablets.
10 to 15 mcgkg oral solution. Additional doses of 125 to 375 mcg may be given at 6 to 8 hour intervals until clinical evidence of an adequate effect is noted.